One of the key features of beer is carbonation. Carbonation has many functions in beer, from enhancing aroma and flavor to increasing beer’s visual appeal to providing a palate-cleansing effect when pairing a beer with a meal. While beer carbonation is simply carbon dioxide dissolved into beer, its use in various beer styles and traditions make it anything but boring.
Table of Contents
Boiled Down Summary
- Beer carbonation is carbon dioxide (CO2) dissolved into the liquid of beer.
- Carbonation is primarily measured in two different ways: by volume at standard temperature and pressure (volumes) and by weight per unit volume (e.g., grams/liter).
- The behavior of beer carbonation and carbon dioxide release in a beer glass can be explained by Henry’s Law, which describes the relationship between the partial pressure of carbon dioxide in the gas phase around a beer and the concentration of dissolved carbon dioxide in the beer.
- Brewers can carbonate beer naturally or artificially through a range of methods.
What is beer carbonation?
Carbonation is simply carbon dioxide (CO2) dissolved into the liquid phase of beer.
Carbon dioxide is a natural product of beer fermentation, wherein fermentable sugars are transformed into primarily carbon dioxide and ethanol. However, the CO2 in beer can come from fermentation or other sources (see Methods of Beer Carbonation below). As with many other beer characteristics, brewers control the amount of carbon dioxide added and the process used to add it to the beer based on beer style, tradition, and other factors.
Carbonation measurement in beer
Carbonation levels in beer are typically measured one of two ways: in volumes or by weight of CO2.
One volume of CO2 is defined as the amount of CO2 that would fill a given volume at standard temperature and pressure (STP). For example, if a pint of beer has two volumes of CO2, it has twice the amount of CO2 that would fit in that same pint volume at STP.
The weight-based method measures the weight of CO2 dissolved per unit of volume, typically in grams/liter (g/L). There is an easy conversion between the two forms: 1 volume CO2 = 1.96 g/L CO2 (Mosher, 2017, p.119).

Typical carbonation levels of beer are between 2.3 and 2.8 volumes (Ockert, 2006, p. 157). However, carbonation levels can range from 4+ volumes for some Belgian ales to less than 1 volume for some British cask ales (Mosher, 2017, p.118).
Carbonation and Henry’s Law
Brewers can control the amount of carbon dioxide in a beer by controlling the amount of carbon dioxide in the gas phase above the beer using Henry’s Law.
Henry’s Law relates the concentration of a gas dissolved in a liquid to the partial pressure of the gas in the gas phase at equilibrium. In the case of carbon dioxide in beer, this can be expressed using the equation from Liger-Belair & Cilindre (2021) outlined in the figure below.
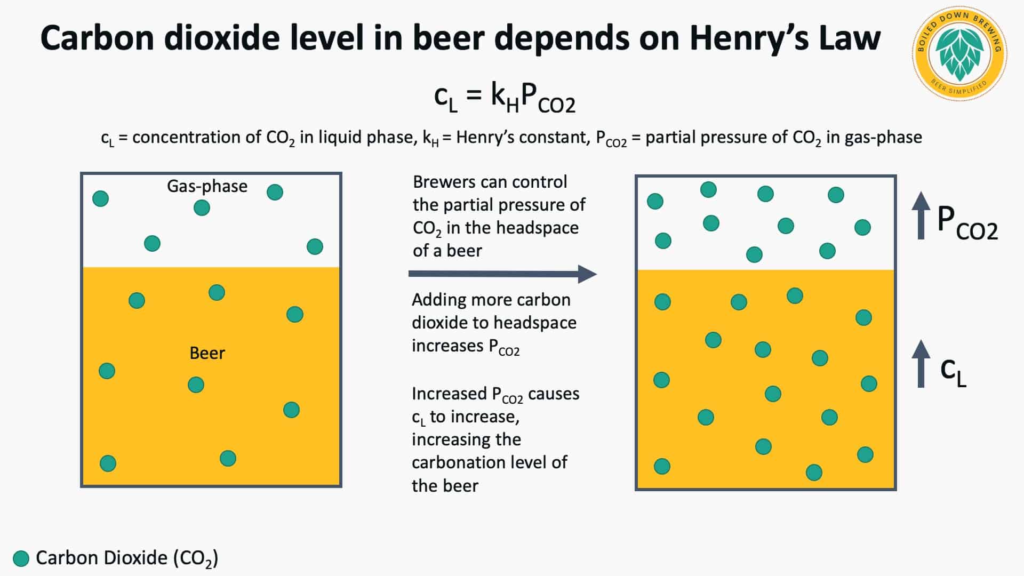
The amount of carbon dioxide in solution is proportional to the partial pressure of carbon dioxide in the gas phase and a temperature-dependent Henry’s constant for carbon dioxide in the beer. In practice, this means that for a given beer at a fixed temperature, the amount of carbon dioxide in the beer (cL) will increase as the partial pressure of carbon dioxide in the gas headspace increases (PCO2).
Brewers can reach their desired concentration of carbon dioxide in a beer with this method by controlling the headspace pressure of carbon dioxide in a vessel. There are charts published for brewers that offer approximations for carbon dioxide solubility in beer at various temperatures, such as the chart found in the MBAA Practical Handbook for the Specialty Brewer Volume 2 (Ockert, 2006 pp. 238-239).
Henry’s Law is also important in maintaining the carbonation levels of beers in draught beer systems. As beers are poured from a keg in a draught beer system, carbon dioxide or a mix of nitrogen and carbon dioxide is added to the headspace of the keg to push the beer.
The partial pressure of carbon dioxide in the headspace needs to be balanced with the concentration of dissolved carbon dioxide in the beer for the beer to stay at the level of carbonation the brewer intended. The Brewers Association has a great free resource called the Draught Beer Quality Manual covering this and many other aspects of serving draught beer.
Why do carbon dioxide bubbles come out of beer?
When finished beer is in bottles, cans, kegs, or other vessels, Henry’s Law equilibrium exists between the carbon dioxide in solution and the small amount of gas headspace in the vessel, keeping the carbon dioxide in the beer. But once the beer is opened, this equilibrium is shifted as the partial pressure of CO2 in the air is much lower than it was in the gas headspace in the closed beer container. For the beer to return to equilibrium with the partial pressure of CO2 in the air again, a large portion of the CO2 must come out of the beer and into the air.
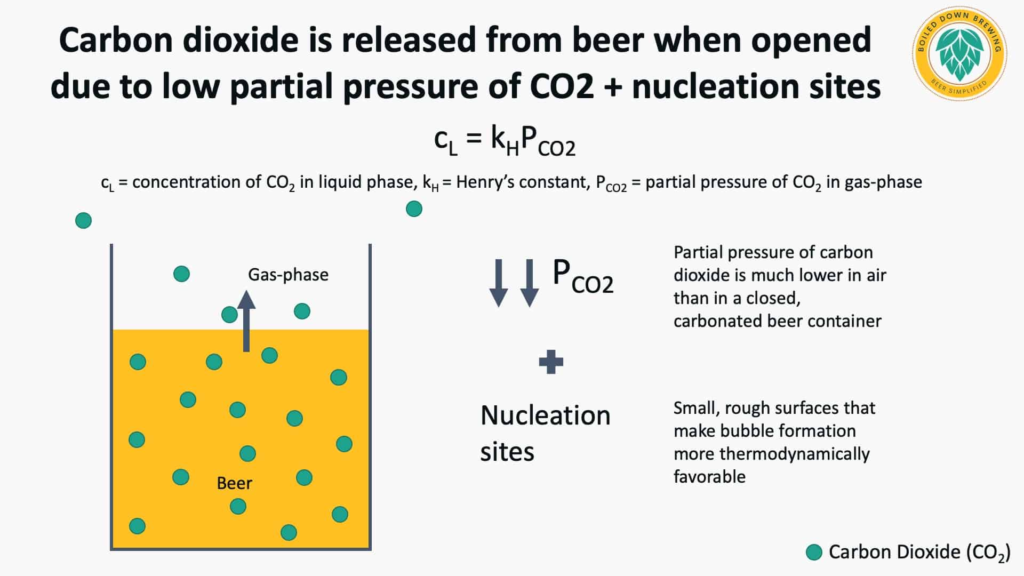
Liger-Belair & Cilindre (2021) outline that at the carbonation levels typically in beer, bubble formation is not likely due to an energy barrier without heterogeneous bubble nucleation. This is caused by small gas cavities in the liquid that can initiate bubble formation. However, most glassware has small crevices that act as initiation points for nucleation.
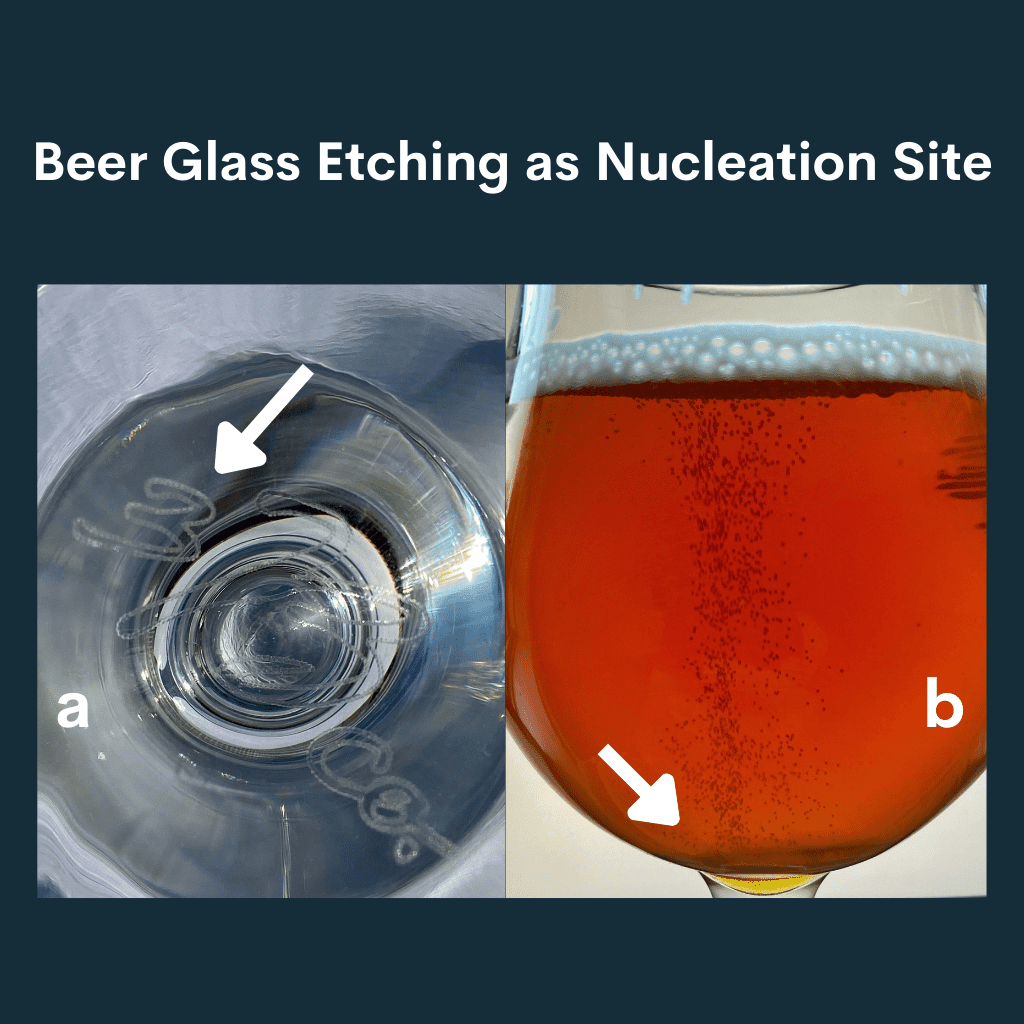
Some beer glasses have designs intentionally laser-etched into the bottom of the glass to provide a nucleation point for bubble formation.
The image below shows an example of a beer glass with a laser-etched logo in the bottom (left) and that same beer glass filled with beer with a visible bubble stream coming from that nucleation point in the glass (right).
Methods of beer carbonation
There are two major methods brewers can use to add CO2 to beer: natural and artificial carbonation.
Natural carbonation
Natural carbonation methods use the CO2 produced by yeast to carbonate the beer. Some of the main natural carbonation methods are covered below.
One method traditionally used in lagers is to control the amount of natural carbon dioxide pressure in a secondary fermentation by using a pressure control valve called spunding in German. The brewer will set the pressure for the valve depending on the desired carbonation level and the temperature of the beer being cellared (Kunze, 2019, p. 419).
Brewers can also recover the carbon dioxide from fermentation, which can be purified and reintroduced to adjust carbonation (Boulton & Quain, 2001, pp. 453-454).
Beer can also be naturally carbonated in the vessel in which the beer will be distributed through refermentation. Cask-conditioned ales and bottle-conditioned beer are examples of this. The Campaign for Real Ale (CAMRA) group based in England is a good resource for learning more about cask-conditioned ales, which you can find here.

Artificial carbonation
Some brewers also carbonate beer using carbon dioxide not produced through their fermentation process. Typically, this type of carbonation is performed either in-line during transfer to the bright beer tank or by carbonation in the bright beer tank by passing carbon dioxide through carbonation stones that create fine CO2 bubbles (Ockert, 2006 pp. 158-159).
References
Boulton, C., & Quain, D. (2001). Brewing Yeast and Fermentation (2nd ed.). Blackwell Sciences Ltd.
Clark, R., Linforth, R., Bealin-Kelly, F., & Hort, J. (2012). Effects of Ethanol, Carbonation and Hop Acids on Volatile Delivery in a Model Beer System. Journal of the Institute of Brewing, 117(1), 74-81. https://doi.org/10.1002/j.2050-0416.2011.tb00446.x
Kunze, W. (2019). Technology Brewing and Malting (6th ed.). VLB Berlin.
Liger-Belair, G., & Cilindre, C. (2021). How Many CO2 Bubbles in a Glass of Beer? ACS Omega, 6(14), 9672-9679. https://doi.org/10.1021/acsomega.1c00256
Mosher, R. (2017). Tasting Beer: An Insider’s Guide to the World’s Greatest Drink (2nd ed.). Storey Publishing.
Ockert, K. (Ed.) (2006). Practical Handbook for the Specialty Brewer: Fermentation, Cellaring, and Packaging Operations Volume 2. Master Brewers Association of the Americas.

