Some ales, particularly those of Belgian origin, are known to be highly attenuated and have a crisp, dry finish. But what makes these beers so highly attenuated compared to other beers also fermented with Saccharomyces cerevisiae?
The answer is a yeast strain capable of producing extracellular glucoamylase, an enzyme that degrades dextrins and soluble starches in beer.
Table of Contents
Boiled Down Summary
- First documented in the 1940s, some strains of Saccharomyces yeasts can produce extracellular glucoamylase, an enzyme capable of degrading starch and dextrins, producing super-attenuated beers.
- These super-attenuating yeast strains were named Saccharomyces diastaticus in the 1950s and considered a new species, but we now know they are Saccharomyces cerevisiae (ale yeast).
- The original focus on super-attenuating yeasts was related to them being beer spoilage organisms that could cause over attenuation in packaging, causing excessive carbon dioxide, draft foaming, bottle gushing, or even bottle bursting.
- The STA1 gene encodes for the extracellular glucoamylase in these high-attenuating strains. Some commercial yeast strains, particularly of Belgian beer origin, are known to be STA1+ strains.
- There are PCR-based methods to test for STA1+ and growth-based methods to test strains for copper tolerance (which STA1+ yeast have) and the ability to grow on starch-based or dextrin-based media.
Discovery of glucoamylase-producing yeasts
Yeast strains capable of degrading soluble starch and dextrins in beer were first described in the 1940s and found to produce beers that were very highly attenuated (Gilliland, 1965).
In the 1950s, these starch-degrading strains were isolated and named Saccharomyces diastaticus due to their ability to produce glucoamylase (also called amyloglucosidase), an enzyme that degrades dextrins and soluble starches by releasing glucose units from the non-reducing end. In the 1950s, S. diastaticus was considered a separate species from traditional ale yeast, S. cerevisiae. However, due to testing developments, we now know that they are both contained in the S. cerevisiae species classification.
Since their discovery in the 1940s, super-attenuating Saccharomyces yeast strains that produce glucoamylase have been studied primarily as beer-contaminant organisms. Accidental introduction of these strains to beer can lead to excessive carbon dioxide production in the bottle, foaming draft beer, bottle gushing, or even bottle bursting (Meier-Dörnberg et al., 2018). Glucoamylase produced by the yeast releases glucose from dextrins or soluble starches in the finished beer, causing the product’s refermentation. More recent research has demonstrated that some classic brewing strains, particularly of Belgian origin, are part of the family of glucoamylase-producing yeasts.
How glucoamylase degrades dextrins and soluble starches in beer
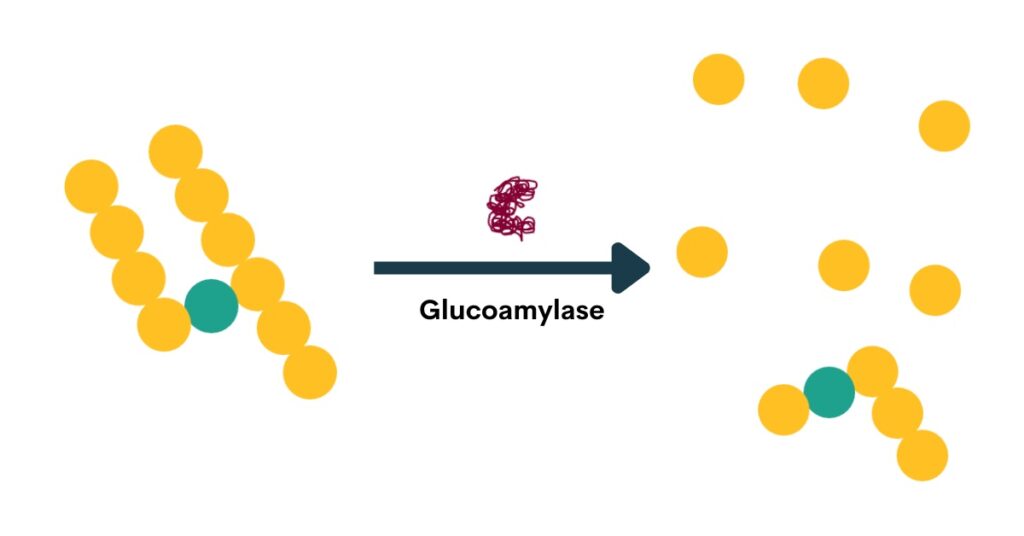
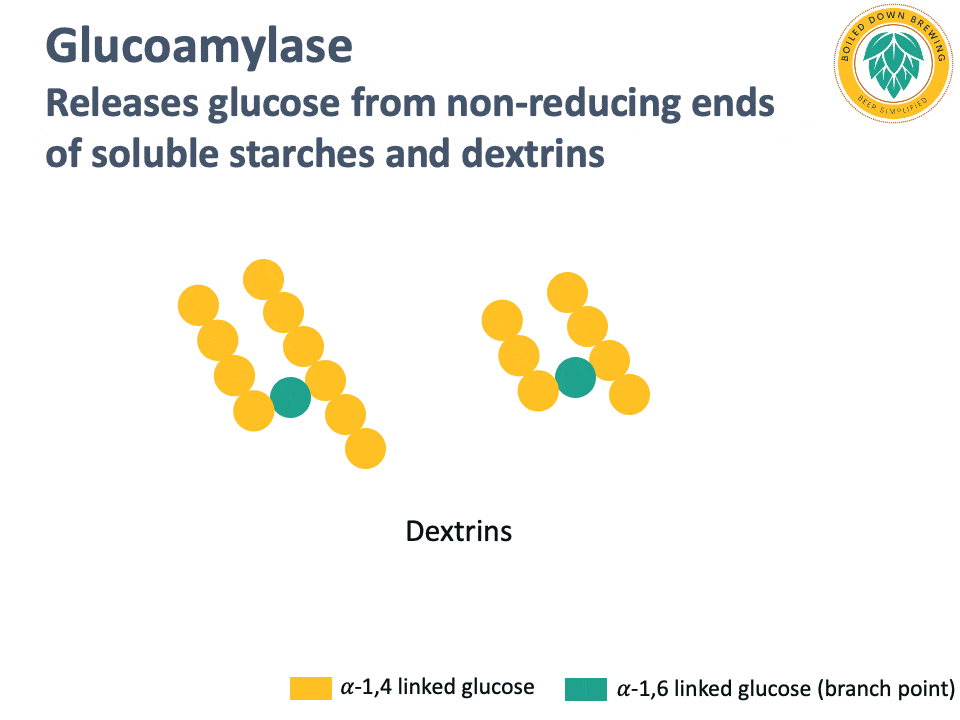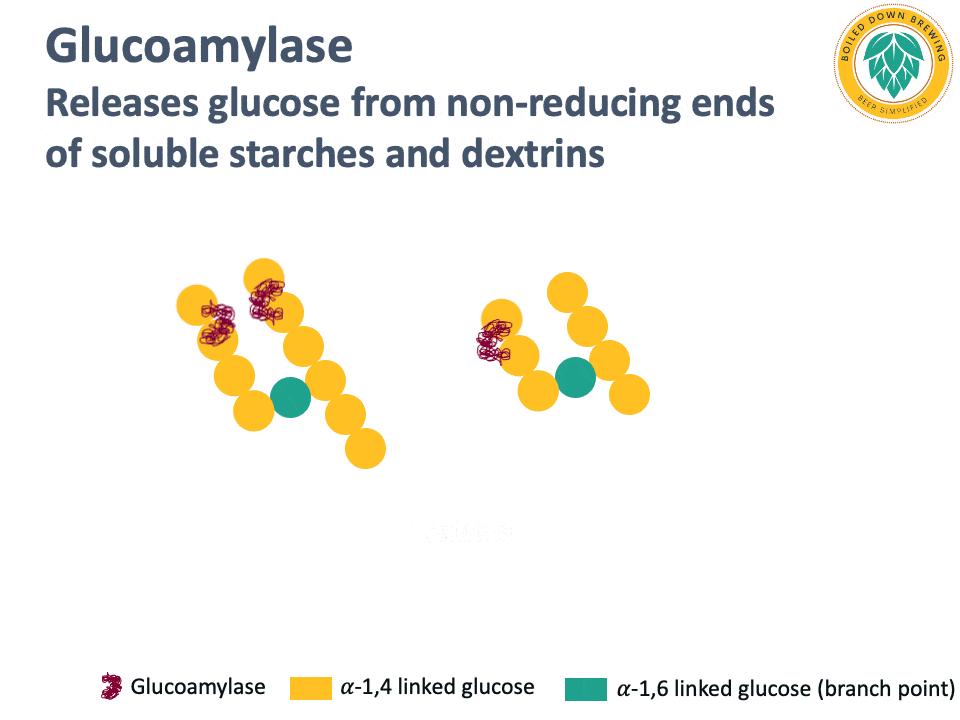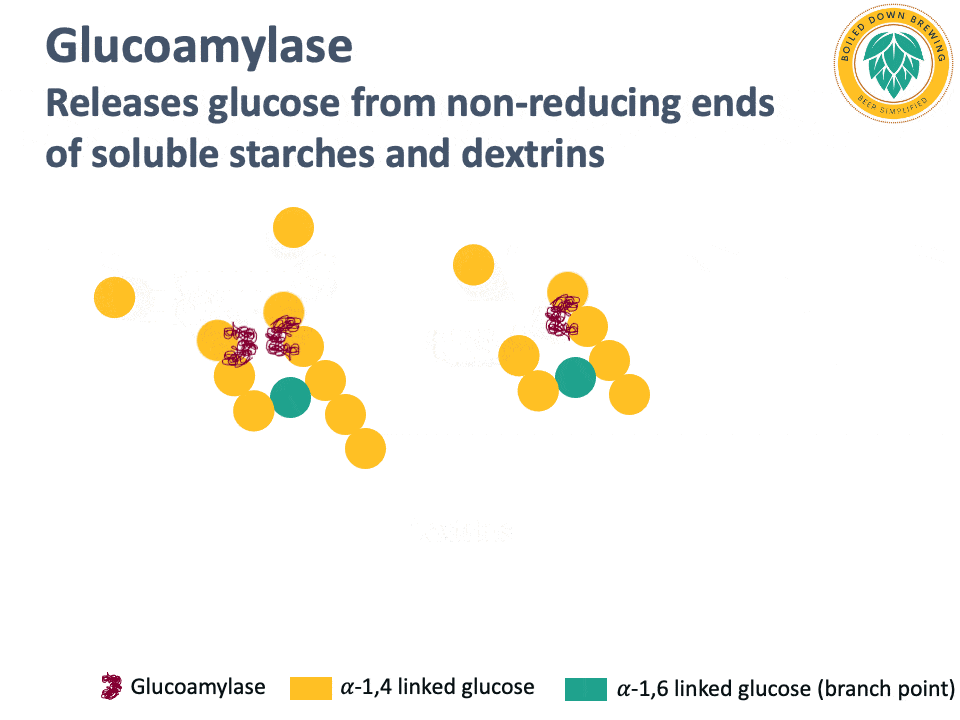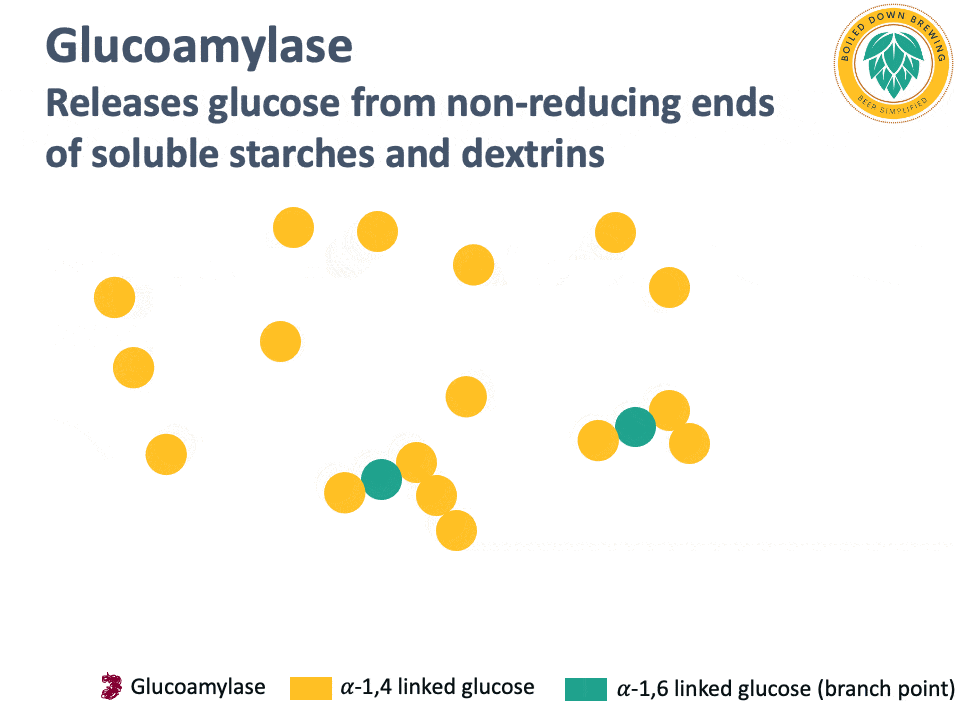
Glucoamylase (also called amyloglucosidase) is an enzyme that hydrolyzes the glycosidic bond at the non-reducing end of starch and dextrin chains, releasing glucose (Kumar & Satyanarayana, 2009). The glucoamylase produced by super-attenuating Saccharomyces yeasts lacks a starch-binding domain, so it cannot degrade native, non-soluble starch (Krogerus & Gibson, 2020). By releasing glucose and degrading dextrins and soluble starches, glucoamylase produces highly attenuated beers.
Below is an animation of the release of glucose from the non-reducing end of dextrins in beer.
Industrial glucoamylases are also used in the brewing industry for making highly attenuated beers.
Traditionally this has been beer styles like light lager, but some recent craft styles, like Brut IPA, also utilize glucoamylase to make ultra-dry beers.
More information on starch-degrading enzymes naturally present in malted barley can be found in this article.
STA1 – the gene that encodes glucoamylase in yeast
The extracellular glucoamylase produced by super-attenuating Saccharomyces cerevisiae strains is encoded by the STA1 gene (Krogerus & Gibson, 2020). Meier-Dörnberg et al. (2018) evaluated 19 spoilage yeast cultures in mostly beer-based beverages and found that 18 of these spoilage yeasts were identified as Saccharomyces cerevisiae var diastaticus (STA1+). They also found that all the STA1+ strains identified were positive for phenolic off-flavor (POF+). Of the 18 identified STA1+ strains, only one could not produce super-attenuated beer.
Krogerus et al. (2020) evaluated why some yeast strains that contain the STA1 gene do not have the super-attenuating ability while others do. They found that some STA1+ yeast strains had a 1162-bp deletion in the STA1 promoter. Strains with the full promoter had a 100-fold higher expression of STA1 (glucoamylase production) than strains with the base pair deletion.
STA1+ yeast strains fall into a category of beer strains called “Beer 2″/” Mosaic Beer” that can utilize maltotriose but does not have AGT1-encoded transporters that bring maltotriose into the yeast cell. Krogerus et al. (2020) hypothesized that the STA1 gene is an evolutionary alternative for the utilization of maltotriose, where glucose is released in the bulk beer by glucoamylase and then utilized by the yeast.
Many Belgian yeast strains are STA1+
Knowing from the work by Meier-Dörnberg et al. (2018) that many STA1+ yeast strains are also phenolic off-flavor positive (POF+), it is not surprising that many Belgian yeast strains are STA1+. Many Belgian beers, particularly farmhouse-style beers like Saison, are known to be highly attenuated, dry beers that have phenolic notes of pepper and spices. White Labs, a producer of brewing yeasts, tests each of its available brewing strains for STA1 and presents the results on its website. At the time of writing this article, most of the products containing STA1+ strains White Labs offers are for producing Belgian-style beers like Saison, Belgian Golden Ale, or mixed fermentation Belgian-style beers.
Methods for testing for super-attenuating Saccharomyces yeasts
Testing for whether a Saccharomyces cerevisiae strain is super-attenuating can be complex. As mentioned previously, the presence of the STA1 gene alone does not indicate whether a strain will be super-attenuating, as some strains have a base pair deletion that makes the expression of STA1 very low.
Growth-based methods can be used to evaluate if a strain is super-attenuating. For example, diastatic yeasts tend to be able to grow on copper-containing media, while typical ale and lager strains cannot. Growth tests on media with starch and dextrin as substrates can also be used, but the downside of all growth methods is that they can take a long time to get results. Krogerus & Gibson (2020) recommend a combination of PCR testing for STA1 and growth-based methods, even though it is time-consuming.
Omega Yeast provides an overview of assays to assess STA1+ yeast strains that is a good summary of currently available methodologies.
References
Gilliland, R. (1965). Saccharomyces Diastaticus – A Starch-Fermenting Yeast. Journal of the Institute of Brewing. https://doi.org/10.1002/j.2050-0416.1966.tb02964.x
Krogerus, K., & Gibson, B. (2020). A re-evaluation of diastatic Saccharomyces cerevisiae strains and their role in brewing. Applied Microbiology and Biotechnology, 104, 3745-3756. https://doi.org/10.1007/s00253-020-10531-0
Kumar, P., & Satyanarayana, T. (2009). Microbial glucoamylases: characteristics and applications. Crit Rev Biotechnol ., 29(3), 225-255. https://doi.org/10.1080/07388550903136076
Meier-Dörnberg, T., Kory, O. I., Jacob, F., Michel, M., & Hutzler, M. (2018). Saccharomyces cerevisiae variety diastaticus friend or foe?—spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Research, 18(4). https://doi.org/10.1093/femsyr/foy023